It now looks like the pharmaceutical market is at a turning point. For more than a century, pharmaceutical companies have been manufacturing their tablets and capsules almost exclusively in batch procedures where individual production stages are processed strictly in succession. This stop-and-go method still remains the most popular way of producing solid formulations today. However, one look at market developments in recent years indicates that the traditional leading position held by the batch process could soon start to falter.
Even today, increasing numbers of oral solid dosage pharmaceuticals (OSD) are being produced using Continuous Manufacturing methods. Since 2015, the Food and Drug Administration (FDA) has approved six pharmaceutical products in tablet form for production with continuous processes, including drugs for the treatment of cancer and cystic fibrosis.
From the manufacturers‘ point of view, one of the decisive arguments for continuous production is the great potential for safe and costsaving processes, whereby the high efficiency of the process plays a significant role. According to the FDA, the international pharmaceutical industry is currently losing up to 50 billion USD a year due to inefficient processes.
Compact and efficient: direct compression
Continuous Manufacturing is based on a new understanding of processes: the product in one process forms the direct base material for the next one. Unlike batch procedures, continuous lines are operated without interruption over a longer period of time. The continuous flow of materials does not require any storage space for intermediate products and makes it possible to scale batch sizes solely over the lines’ runtime. This process also facilitates swift market launches of new products as the same line can be used for both development and production.
As a general rule, the more streamlined the design of the continuous line, the more efficient the production process. For this reason, Fette Compacting relies on the compact set-up of direct compression for its continuous tableting lines.
After the dosing process, the powder is fed directly from the mixer into the tablet press without any additional granulation. If required, the tablets can also be coated afterwards. This dispenses with several cost-intensive production steps resulting from the usual granulation processes, which are often necessary in batch production. This streamlining of the process is made possible by an uninterrupted material flow with consistently high product quality.

Fette Compacting retains an entire test line for direct compression in the Competence Center in Schwarzenbek where customers can run product tests, trial individual components, or adapt the complete production line to their own requirements.
Quality control in real time
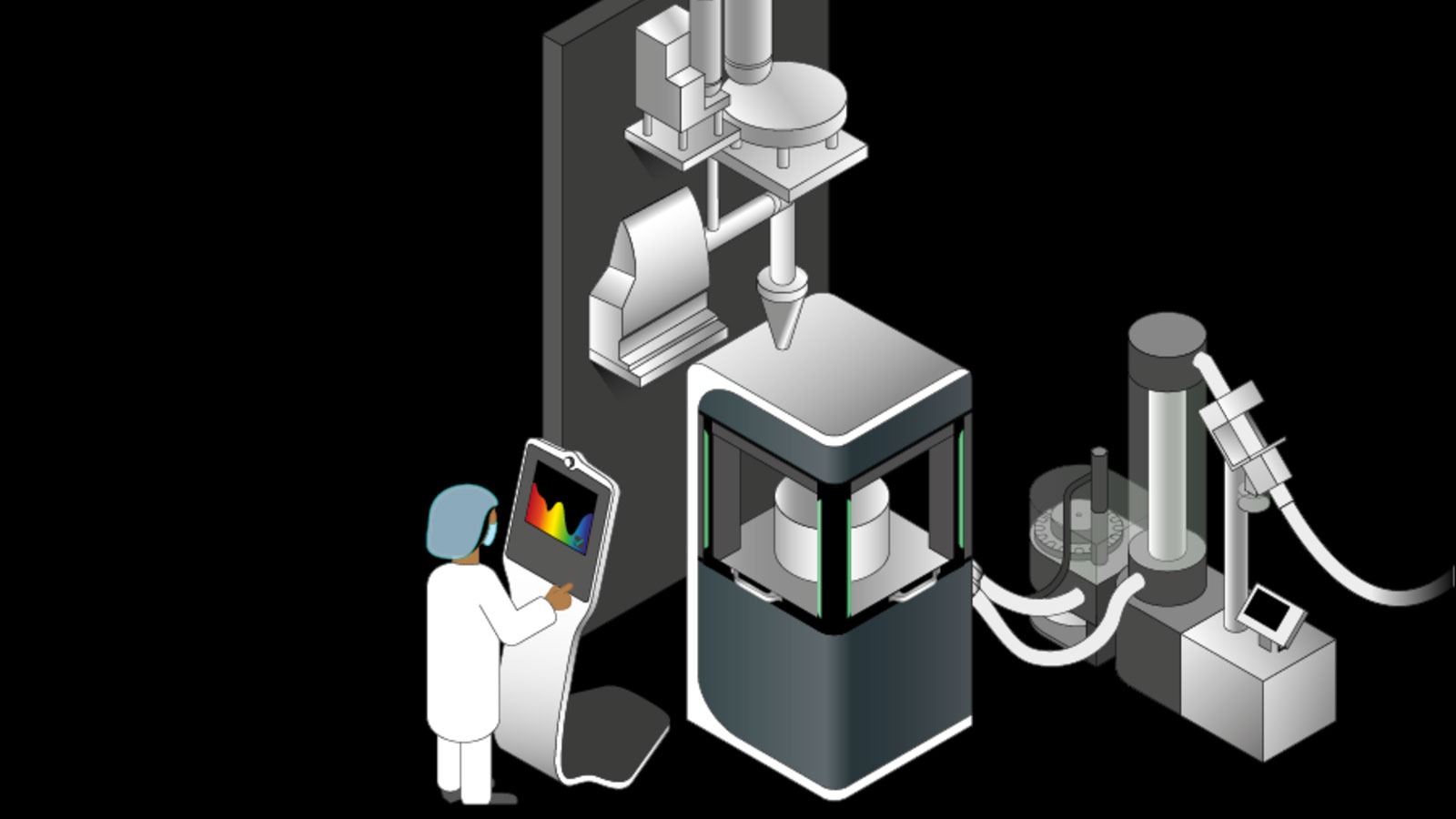
Because direct compression only takes a few steps, it requires minimum equipment which keeps the space requirements low compared to other continuous processes. Quality-reducing factors such as vibrations during transport of intermediate products which can lead to segregation can also be significantly reduced.
The direct compression lines supplied by Fette Compacting are based on the efficient tablet presses of the FE Series. Together with a horizontal mixing system and high precision dosing units, these form a safe and efficient continuous system for a wide range of capacities.
The constant high quality of products is guaranteed by highly-developed PAT (Process Analytical Technology) sensors for which Fette Compacting primarily relies on near infrared spectroscopy (NIR). NIR sensors in the tablet presses analyze in real-time each tablet in terms of its concentration of active ingredient. This makes it possible to optimize the production process using the data gleaned and to ensure the quality of the finished tablets.
Where the NIR sensors reach their performance limits, Raman spectroscopy is applied to determine even minute concentration of active ingredient using its powerful laser. In special cases, laserinduced fluorescence (LIF), UV, or terahertz spectroscopy can also be relied on.
Please do not hesitate to contact us if you have any queries.





